2017.HSA.doxorubicin.AC.vs.ADTIC
(→治疗方案 (Treatment protocol)) |
(→治疗方案 (Treatment protocol)) |
||
| 第67行: | 第67行: | ||
| − | ''' | + | '''标准止吐治疗''' 从化疗第一天开始连续 3 天以 2 mg/kg q24h 的剂量口服'''马罗匹坦 (maropitant)'''(赛瑞宁 Cerenia®、Pfizer、Latina、Italy)。 |
'''克拉维酸增强阿莫西林'''(Synulox®,辉瑞,拉丁,意大利)以 12.5-20 mg/kg q12h 的频率预防性口服给药,直到预期的中性粒细胞减少性最低点。抗生素剂量取决于临床医生的偏好。 | '''克拉维酸增强阿莫西林'''(Synulox®,辉瑞,拉丁,意大利)以 12.5-20 mg/kg q12h 的频率预防性口服给药,直到预期的中性粒细胞减少性最低点。抗生素剂量取决于临床医生的偏好。 | ||
2024年6月26日 (三) 11:45的版本
多柔比星-环磷酰胺与多柔比星-达卡巴肼辅助治疗犬血管肉瘤的比较
Comparison of doxorubicin-cyclophosphamide with doxorubicin-dacarbazine for the adjuvant treatment of canine hemangiosarcoma
https://doi.org/10.1111/vco.12139
目录 |
1 摘要
犬血管肉瘤 (HSA) 是一种血管内皮起源的肿瘤,具有侵袭性生物学行为,在诊断后 12 个月内存活的狗不到 10%。首选的治疗包括手术,然后以阿霉素为基础的辅助化疗。我们前瞻性地比较了阿霉素和达卡巴嗪(ADTIC)辅助治疗与传统的多柔比星和环磷酰胺(AC)治疗,旨在确定安全性并评估该方案是否能延长生存期和转移时间(TTM)。招募了 27 只狗;分期检查后,18 例接受 AC 治疗,9 例接受 ADTIC 治疗。与接受AC治疗的狗相比,接受ADTIC治疗的狗的中位TTM和生存时间更长(分别为>550天和112天,P=0.021和>550天和142天,P=0.011)。两种方案的耐受性都很好,不需要减少剂量或增加治疗间隔。由多柔比星和达卡巴嗪联合组成的方案在患有 HSA 的狗中是安全的,并延长了 TTM 和生存时间。
Canine hemangiosarcoma (HSA) is a neoplasm of vascular endothelial origin that has an aggressive biological behaviour, with less than 10% of dogs alive at 12-months postdiagnosis. Treatment of choice consists of surgery followed by adjuvant doxorubicin-based chemotherapy. We prospectively compared adjuvant doxorubicin and dacarbazine (ADTIC) to a traditional doxorubicin and cyclophosphamide (AC) treatment, aiming at determining safety and assessing whether this regimen prolongs survival and time to metastasis (TTM). Twenty-seven dogs were enrolled; following staging work-up, 18 were treated with AC and 9 with ADTIC. Median TTM and survival time were longer for dogs treated with ADTIC compared with those receiving AC (>550 versus 112 days, P = 0.021 and >550 versus 142 days, P = 0.011, respectively). Both protocols were well tolerated, without need for dose reduction or increased interval between treatments. A protocol consisting of combined doxorubicin and dacarbazine is safe in dogs with HSA and prolongs TTM and survival time.
2 介绍
3 材料与方法 (Materials and methods)
患者资格 (Patient eligibility)
前瞻性地招募了由任何腹部器官或皮下引起的具有手术切除、经组织学证实的 HSA 的客户拥有的狗。术前检查包括体格检查、血液学、血清生化、腹部超声和至少两次胸部 X 线片的侧视图。如果使用超声心动图确定的收缩期缩短分数为 <25%,则认为狗发生多柔比星相关心脏毒性的高风险。具有这种心脏功能的狗没有参加这项研究。患有与 HSA 不同的限制生命的疾病的狗和患有真皮 HSA 的狗也被排除在外。根据世界卫生组织 (WHO) 的家畜分期系统对狗进行分期[29]。第1组包括提及一位作者(D.S.)机构的狗,而提及两位不同作者(L.M.和R.F.)机构的狗被列入第 2 组。
Client-owned dogs with a surgically removed, histologically confirmed HSA, arising from any abdominal organ or subcutis, were prospectively recruited. Presurgical investigations included physical examination, haematology, serum biochemistry, abdominal ultrasound and at least two lateral views thoracic radiographs. Dogs were considered to be at high risk of developing doxorubicin-related cardiotoxicity if systolic fractional shortening determined by using echocardiography was <25%. Dogs with such cardiac function were not enrolled in this study. Dogs with life-limiting diseases different than HSA and those with dermal HSA were also excluded. Dogs were staged according to the World Health Organization (WHO) staging system for domestic animals.29. Dogs referred to the institution of one author (D. S.) were included in group 1, whereas dogs referred to the institutions of two different authors (L. M. and R. F.) were included in group 2.
3.1 治疗方案 (Treatment protocol)
目的是在手术干预后 7-10 天内开始化疗。第 1 组的狗接受辅助多柔比星 (Doxorubicina, Ebewe Italia s.r.l., Roma, Italy) 和环磷酰胺 [Endoxan®, Baxter s.r.l., Lurago d'Erba, Como, Italy (AC)] 治疗,而第 2 组的狗接受辅助多柔比星和达卡巴嗪 [Deticene®, Aventis Pharma S.p.A, Milano, Italy (ADTIC)]。与 AC 相比,ADTIC 更昂贵,并且由于该研究没有得到资助,因此无法随机分组。由于与传统的 AC 协议相比,ADTIC 在进行本研究时完全处于研究阶段,因此所有选择 ADTIC 的业主都被要求在注册前签署书面知情同意书。
The objective was to initiate chemotherapy within 7–10 days after surgical intervention. Dogs included in group 1 were treated with adjuvant doxorubicin (Doxorubicina, Ebewe Italia s.r.l., Roma, Italy) and cyclophosphamide [Endoxan®, Baxter s.r.l., Lurago d’Erba, Como, Italy (AC)], whereas dogs included in group 2 received adjuvant doxorubicin and dacarbazine [Deticene®, Aventis Pharma S.p.A, Milano, Italy (ADTIC)]. ADTIC was more expensive compared with AC, and as the study was not supported by a grant, groups could not be randomized. Because ADTIC was completely investigational at the time this study was carried out compared with the traditional AC protocol, all owners electing ADTIC were asked to sign a written informed consent prior to enrolment.
在任一治疗组中,阿霉素以 30 mg/m^2 的剂量静脉注射,每 3 周一次,持续四个周期。阿霉素在 20 分钟内以缓慢静脉推注给药。
In either treatment groups, doxorubicin was administered IV at the dose of 30 mg/m^2 every 3 weeks for four cycles. Doxorubicin was administered as a slow IV bolus within 20 min.
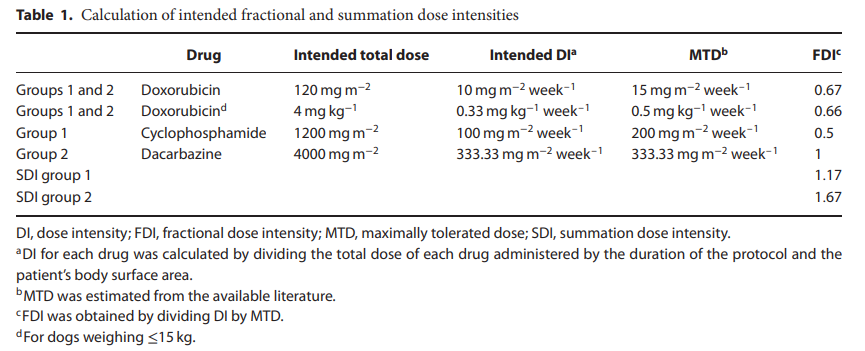
在第 1 组中,环磷酰胺以 75 mg/m^2 口服给药,连续 4 天,从每次阿霉素给药当天开始。在第 2 组中,达卡巴嗪以 200 mg/m^2 的剂量静脉注射(每天不超过 250 mg),每天一次,持续 5 天,从每次多柔比星给药之日开始。简言之,将达卡巴嗪与注射用水复溶,以获得10mg/mL的浓度。然后通过先前放置的留置导管静脉输注 1 分钟以上给药复溶药物,以给予多柔比星。然后移除导管,并在接下来的四次给药中反复放置新导管。表1.30列出了两种方案的分数和总和剂量强度(SDI)
In group 1, cyclophosphamide was administered orally at 75 mg/m^2 for 4 consecutive days, starting on the day of every doxorubicin administration. In group 2, dacarbazine was administered IV at the dose of 200 mg/m^2 (without exceeding 250 mg total daily) once daily for 5 days, starting on the day of every doxorubicin administration. Briefly, dacarbazine was reconstituted with water for injection to obtain a concentration of 10 mg mL−1. Reconstituted drug was then given by intravenous infusion over 1 min via the indwelling catheter previously placed to administer doxorubicin. The catheter was then removed, and a new catheter was repeatedly placed for the following four administrations. Fractional and summation dose intensities (SDIs) for the two protocols are listed in Table 1.[30]
在任一组中,标准止吐治疗包括从化疗第一天开始连续 3 天以 2 mg/kg q24h 的剂量口服马罗匹坦 (maropitant)(赛瑞宁 Cerenia®、Pfizer、Latina、Italy)。克拉维酸增强阿莫西林(Synulox®,辉瑞,拉丁,意大利)以 12.5-20 mg/kg q12h 的频率预防性口服给药,直到预期的中性粒细胞减少性最低点,此后如图所示。抗生素剂量取决于临床医生的偏好。
In either group, standard antiemetic therapy consisted in maropitant (Cerenia®, Pfizer, Latina, Italy) administered orally at the dose of 2 mg kg−1 q24h for 3 consecutive days starting on the first day of chemotherapy. Clavulanate-potentiated amoxicillin (Synulox®, Pfizer, Latina, Italy) was prophylactically administered orally at 12.5–20 mg kg−1 q12h until the time of the expected neutropenic nadir, and as indicated thereafter. Antibiotic dosage depended on clinician’s preference.
在两个周期的化疗后,进行了重复的临床分期检查,包括胸片和腹部超声检查。如果没有观察到局部复发和/或转移性疾病,则将相同的化疗方案继续两个周期。在疾病进展的情况下,提供了抢救方案。随访重新分期包括在方案结束后 1 个月和之后每 3 个月进行一次胸片和腹部超声检查,以确定对治疗的反应。
A repeated clinical staging work-up consisting of thoracic radiographs and abdominal ultrasoundo was performed after two cycles of chemotherapy. If no local recurrence and/or metastatic disease were observed, the same chemotherapy protocol was continued for two additional cycles. In case of disease progression, a rescue protocol was offered. Follow-up re-staging consisted of thoracic radiographs and abdominal ultrasound performed 1 month after the end of the protocol and every 3 months afterwards to define response to treatment.
NOTES:
Group 1 & 2: 多柔比星 30 mg/m^2 的剂量静脉注射,每 3 周一次,持续 4 个周期。多柔比星在 20 分钟内以缓慢静脉推注给药。 Group 1: 环磷酰胺以 75 mg/m^2 口服给药,连续 4 天,从每次多柔比星给药当天开始。每个疗程总剂量为 75x4 = 300 mg/m^2 Group 2: 达卡巴嗪以 200 mg/m^2 的剂量静脉注射(每天不超过 250 mg),每天一次,持续 5 天,从每次多柔比星给药之日开始。 将达卡巴嗪与注射用水复溶,以获得10mg/mL的浓度。然后通过先前放置的留置导管静脉输注 1 分钟以上给药复溶药物,以给予多柔比星。然后移除导管,并在接下来的四次给药中反复放置新导管。 '''标准止吐治疗''' 从化疗第一天开始连续 3 天以 2 mg/kg q24h 的剂量口服'''马罗匹坦 (maropitant)'''(赛瑞宁 Cerenia®、Pfizer、Latina、Italy)。 '''克拉维酸增强阿莫西林'''(Synulox®,辉瑞,拉丁,意大利)以 12.5-20 mg/kg q12h 的频率预防性口服给药,直到预期的中性粒细胞减少性最低点。抗生素剂量取决于临床医生的偏好。
3.2 毒性评估 (Assessment of toxicity)
根据第 7-10 天和每个周期开始前进行的狗的病史、体格检查和全血细胞计数 (CBC),在第 1 组中评估化疗产生的毒性,如兽医合作肿瘤学组所述.31 在第 2 组中,在每个化疗周期的第 1、4、5 和 10 天检查 CBC。第 1 天被认为是阿霉素给药的日子。
Toxicity resulting from chemotherapy was assessed in group 1 based on the dog’s history, physical examination and complete blood count (CBC) performed 7–10 days after doxorubicin and before the beginning of each cycle, as stated by the Veterinary Co-operative Oncology Group.31 In group 2, CBC was checked on days 1, 4, 5 and 10 of each chemotherapy cycle. Day 1 was considered as the day of doxorubicin administration.
3.3 统计分析 (Statistical analysis)
从诊断之日到最后一次就诊或死亡之日的随访和生存时间是根据在作者所在机构进行的复查计算的。对于两组,使用 Kaplan-Meier 乘积限值方法探索生存时间和 TTM(超出区域淋巴结),然后进行对数秩检验。在两组中,手术切除的时机都被认为是。在生存分析中,如果狗在数据累积关闭时还活着或死于没有肿瘤相关原因,则对狗进行审查,而对于TTM狗,如果在最后一次检查中没有发生远处转移,则被审查。
Follow-up and survival times were calculated from the date of diagnosis to the date of last visit or death based on the rechecks performed at one of the authors’ institutions. For both groups, survival time and TTM (beyond regional lymph nodes) were explored using the Kaplan–Meier product limit method followed by log-rank test. In either group, timing was considered from surgical excision. In the survival analysis, dogs were censored if they were alive at the time of data accrual closure or died of no tumour-related causes, whereas for TTM dogs were censored if, by the last examination, distant metastases had not developed.
为了验证两个治疗组的特征在入院时是否不同,使用 Mann-Whitney 检验比较年龄和体重,并使用 Fisher 精确检验比较品种(纯种与杂交)、性别(雄性与雌性)、肿瘤的主要位置(脾脏与其他部位)、临床分期(II 与 III)和手术切缘(完全与不完全)。如果狗出现内脏破裂,则认为手术切缘不可评估。Fisher 精确测试还用于比较治疗周期中发生的骨髓毒性(存在与不存在)的频率。P <0.05 被认为是显著的。
To verify whether characteristics of the two treatment groups differed at admission, the Mann–Whitney test was used to compare age and body weight, and the Fisher’s exact test was used to compare breed (pure- versus cross-breed), sex (male versus female), primary location of the tumour (spleen versus other sites), clinical stage (II versus III) and surgical margins (complete versus incomplete). Surgical margins were deemed not assessable if the dog was presenting with visceral rupture. The Fisher’s exact test was also used to compare the frequency of bone marrow toxicity (present versus absent) that occurred during treatment cycles. P <0.05 was considered significant.
3.4 结论 (Results)
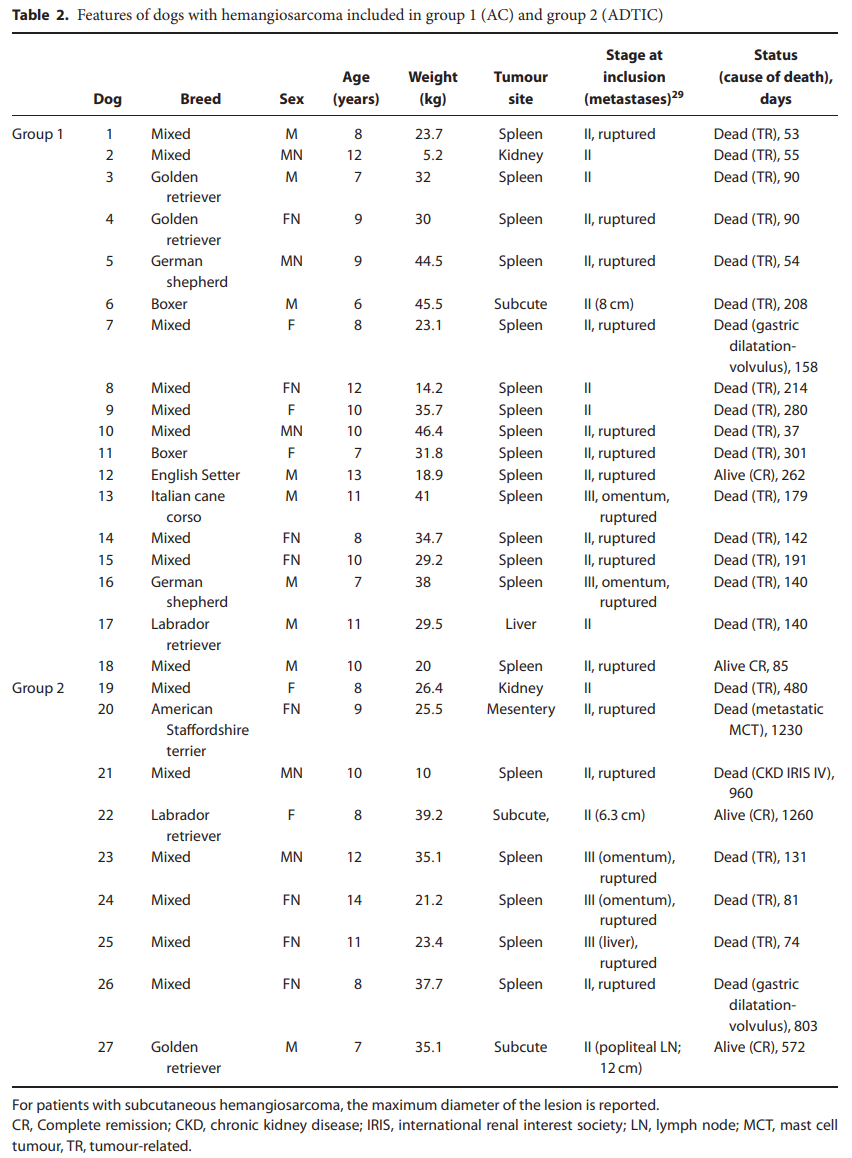
在2008年至2014年期间,有27只狗符合纳入标准并被招募:其中18只(66.6%)接受了辅助AC(第1组),而其余9只(33.3%)接受了ADTIC治疗(第2组)。狗的特征列于表2。
Between 2008 and 2014, 27 dogs met the inclusion criteria and were enrolled: 18 (66.6%) of them received adjuvant AC (group 1), whereas the remaining 9 (33.3%) were treated with ADTIC (group 2). Features of the dogs are listed in Table 2.
入院时,两个治疗组在年龄、体重、品种、性别、肿瘤的主要位置和分期方面没有差异,而分配接受ADTIC的狗的手术切缘往往不完全,而不是接受AC的狗[分别为9例中的5例(55.6%)和18例中的2例(11.1%);P =0.024]。
At admission, the two treatment groups did not differ for age, body weight, breed, sex, primary location of the tumour and stage, whereas surgical margins were more often incomplete in dogs allocated to receive ADTIC than in those allocated to receive AC [5 of 9 (55.6%) versus 2 of 18 (11.1%), respectively; P =0.024].
第 1 组
有九只混种犬,两只拳击手,两只德国牧羊犬,两只金毛猎犬,一只英国二传手,一只拉布拉多猎犬和一只意大利甘蔗犬。中位年龄为 9.5 岁(范围:6-13 岁),中位体重为 31 公斤(范围:5.2-46.4 公斤)。有 11 只雄性(n=3 只绝育)和 7 只雌性狗(n=4 只绝育)。15 只狗的原发部位是脾脏,其中 11 只因脾破裂而出现腹血。其余三只狗分别患有肾脏、肝脏和皮下 HSA。
根据癌症位置,每只狗都接受了手术,包括脾切除术、左肝叶切除术、肾切除术或切除皮下肿瘤。组织病理学评估显示肝脏和皮下 HSA 的手术切缘干净。
根据 TNM 分类,16 只狗被认为患有 II 期,2 只患有 III 期 HSA。两只患有III期疾病的狗都有脾脏HSA和网膜转移的宏观证据;在腹腔切开术中怀疑转移性疾病,并通过组织病理学检查证实。观察到多发性粟粒病变,无法进行转移灶切除术。没有区域淋巴腺肿大和/或其他转移部位的记录。表2总结了案例。
从手术到化疗开始的中位时间为 9 天(范围:7-10 天)。化疗周期的中位数为 4 个(范围:2-5),多柔比星的中位累积剂量为 120 mg/m^2,环磷酰胺的中位累积剂量为 1200 mg/m^2。该协议接受的 SDI 中位数与预期的 SDI 相对应,因为没有一只狗需要减少剂量和/或延迟治疗。
Group 1
There were nine mixed breed dogs, two Boxer, two German shepherd, two Golden retrievers, one English setter, one Labrador retriever and one Italian cane corso. Median age was 9.5 years (range: 6–13 years) and median weight was 31 kg (range: 5.2–46.4 kg). There were 11 males (n=3 neutered) and 7 female dogs (n=4 spayed). HSA occurred in the spleen as primary site in 15 dogs: 11 of them presented with hemoabdomen because of splenic rupture. The remaining three dogs had a renal, hepatic and a subcutaneous HSA, respectively.
Each dog underwent surgery, consisting of splenectomy, left hepatic lobectomy, nephrectomy or removal of the subcutaneous tumour, according to cancer location. Histopathological evaluation revealed clean surgical margins in the hepatic and subcutaneous HSA.
According to the TNM classification, 16 dogs were considered having stage II and 2 having stage III HSA. Both dogs with stage III disease had a splenic HSA and macroscopic evidence of metastasis to the omentum; metastatic disease was suspected during celiotomy, and this was confirmed through histopathological examination. Multiple miliary lesions were observed, and metastasectomy could not be performed. No regional lymphadenomegaly and/or other metastatic sites could be documented. Cases are summarized in Table 2.
The median time from surgery to the initiation of chemotherapy was 9 days (range: 7–10 days). The median number of chemotherapy cycles was 4 (range: 2–5), with a median cumulative dose of doxorubicin of 120 mg m–2 and a median cumulative dose of cyclophosphamide of 1200 mg m−2. The median received SDIs for this protocol corresponded to the intended SDIs, as none of the dogs required dose reductions and/or treatment delays.
第 2 组
有六只混种犬,一只美国斯塔福德郡梗犬,一只金毛猎犬和一只拉布拉多猎犬。中位年龄为 9 岁(范围:8-14 岁),中位体重为 26.4 公斤(范围:10-39.2 公斤)。有三只雄性(n=1 绝育)和六只雌性狗(n=4 绝育)。HSA发生在5只狗的脾脏中,是主要部位;所有患者均因脾破裂而出现腹腔积血。两只狗有皮下HSA,一只狗有肾,一只有肠系膜HSA。根据TNM分类,6只狗患有II期HSA(n=2脾脏,n=2皮下,n=1肾和n=1肠系膜),3只狗患有III期HSA(n=3脾脏)。一只患有皮下 II 期 HSA 的狗患有同侧区域淋巴结淋巴腺肿大;手术切除,组织病理学证实转移性疾病。患有III期疾病的三只狗中有两只有腹膜转移,一只有肝转移。所有狗都接受了手术,包括脾切除术、肾切除术、肠系膜和皮下肿瘤切除术,具体取决于癌症位置;在所有III期疾病病例中,由于转移灶数量众多,无法进行转移灶切除术。切除两个边缘不完整的皮下 HSA。表2总结了案例。
从手术到化疗开始的中位时间为 9 天(范围:7-10 天)。化疗周期的中位数为 4 个(范围:2-4 个周期),阿霉素的中位累积剂量为 120 mg/m^2(范围:60-120 mg/m^2,达卡巴嗪的中位累积剂量为 4000 mg/m^2(范围:2000-4000 mg/m^2)。化疗周期的中位数为4个(范围:2-4)。该协议接收的 SDI 中位数与预期的 SDI 相对应。
Group 2
There were six mixed breed dogs, one American Staffordshire terrier, one Golden retriever and one Labrador retriever. Median age was 9 years (range: 8–14 years) and median weight was 26.4 kg (range: 10–39.2 kg). There were three males (n=1 neutered) and six female dogs (n=4 spayed). HSA occurred in the spleen as primary site in five dogs; all of them presented with hemoperitoneum because of splenic rupture. Two dogs had subcutaneous HSA, one dog had a renal and one had a mesenteric HSA. According to the TNM classification, six dogs had stage II HSA (n=2 splenic, n=2 subcutaneous, n=1 renal and n=1 mesenteric), and three dogs had stage III HSA (n=3 splenic). One dog with subcutaneous stage II HSA had lymphadenomegaly of the ipsilateral regional lymph node; this was surgically excised, and metastatic disease was confirmed on histopathology. Two of the three dogs with stage III disease had peritoneal metastases, and one had liver metastases. All dogs underwent surgery, consisting of splenectomy, nephrectomy, removal of mesenteric and subcutaneous tumour, according to cancer location; in all cases with stage III disease, metastasectomy was not possible because of the multiple number of metastases. Two subcutaneous HSA were removed with incomplete margins. Cases are summarized in Table 2.
The median time from surgery to the initiation of chemotherapy was 9 days (range: 7–10 days). The median number of chemotherapy cycles was 4 (range: 2–4 cycles), with a median cumulative dose of doxorubicin of 120 mg m−2 (range: 60–120 mg m−2) and a median cumulative dose of dacarbazine of 4000 mg m−2 (range: 2000–4000 mg m−2). The median number of chemotherapy cycles was 4 (range: 2–4). The median received SDIs for this protocol corresponded to the intended SDIs.
3.5 其他治疗 (Additional treatments)
在发生转移性疾病时允许进行额外的治疗。这些只在三只狗身上建立。在第 1 组中,患有脾 II 期 HSA 的狗在节律方案中接受环磷酰胺和吡罗昔康,在第 2 组中,患有 II 期肾 HSA 的狗和患有脾 III 期 HSA 的狗接受异环磷酰胺,然后接受甲磺酸马赛替尼。
Additional treatments were permitted at the time of development of metastatic disease. These were instituted only in three dogs. In group 1, a dog with splenic stage II HSA received cyclophosphamide and piroxicam in a metronomic regimen, and in group 2, a dog with stage II renal HSA and one with splenic stage III HSA received ifosfamide followed by masitinib mesylate.
3.6 临床结果 (Clinical outcomes)
第 1 组的 18 只狗中有 13 只 (72.2%) 在中位 89 天(范围:44-188 天)后出现转移性疾病。在肝脏(n=7)、肺(n=3)、腹膜(n=2)、肝脏和肺(n=1)中发现转移。两只腹膜转移的狗出现了腹腔血肿。第 2 组的 9 只狗中有 1 只 (11.1%) 在 378 天后发生肺转移。在就诊时已经发生转移的三只狗中,有两只分别在 48 天和 70 天后记录了疾病进展。在这些狗中,分别在肺(n = 1),肾脏和肝脏(n = 1)中发现了转移。总体而言,与接受 AC 的狗相比,接受 ADTIC 的狗使用 Kaplan-Meier 产品限值计算的中位 TTM 显着更长(分别为 >550 天和 112 天;P =0.021;图1)。第 1 组中包括 18 只狗的 16 只 (88.8%) 在研究结束时死亡:15 只 (83.3%) 因 HSA 进展而死亡,中位生存时间为 140 天(范围:37-301 天),而一只狗在 158 天后死亡,原因是
Thirteen (72.2%) of the eighteen dogs in group 1 developed metastatic disease after a median of 89 days (range: 44–188 days). Metastases were found in the liver (n=7), lungs (n=3), peritoneum (n=2), liver and lungs (n=1). The two dogs with metastases to the peritoneum developed haemoabdomen. One (11.1%) of the nine dogs included in group 2 developed pulmonary metastasis after 378 days. Of the three dogs already having metastasis at presentation, two had disease progression documented after 48 and 70 days, respectively. In these dogs, metastases were found in the lungs (n=1), kidney and liver (n=1), respectively. Overall, median TTM as calculated with Kaplan–Meier product limit was significantly longer for dogs receiving ADTIC compared with those receiving AC (>550 days versus 112 days, respectively; P =0.021; Fig. 1). Sixteen (88.8%) of the eighteen dogs included in group 1 died by the end of the study: 15 (83.3%) died as a result of HSA progression with a median survival time of 140 days (range: 37–301 days), whereas one dog died after 158 days because of
胃扩张-扭转,无肿瘤复发或转移的证据。两只患有脾 II 期 HSA 的狗在诊断后分别存活 85 天和 262 天。第 2 组的 9 只狗中有 7 只 (77.7%) 在研究结束时死亡:4 只 (44.4%) 因 HSA 进展而死亡,中位生存时间为 106 天(范围:74-480 天)。在这四只狗中,三只患有脾脏 III 期 HSA,一只患有肾脏 II 期 HSA。其余3例(脾II.期HSA)在诊断后803天因胃扩张-扭转死亡,1例(脾II.期HSA)960天后死于晚期慢性肾病[国际肾脏协会(IRIS)IV期],1例(肠系膜II.期HSA)在1230天后死于转移性肥大细胞瘤。两只患有皮下 II 期 HSA 的狗在诊断后 572 天和 1260 天仍然活着。总体而言,接受ADTIC的狗的中位生存期明显长于接受AC的狗(分别为>550天和142天;P =0.011;图2)。在第 1 组中,没有一只狗在诊断后 1 年存活,而在第 2 组中,1 年和 1.5 年生存率分别为 66.8% 和 55.8%
gastric dilatation-volvulus with no evidence of tumour recurrence or metastasis. Two dogs with splenic stage II HSA were still alive, 85 and 262 days after the diagnosis, respectively. Seven (77.7%) of the nine dogs in group 2 died by the end of the study: four (44.4%) died as a result of HSA progression with a median survival time of 106 days (range: 74–480 days). Of these four dogs, three had splenic stage III HSA and one had renal stage II HSA. Regarding the remaining three, one of them (splenic stage II HSA) died 803 days after the diagnosis owing to gastric dilatation-volvulus, one (splenic stage II HSA) died after 960 days owing to advanced chronic kidney disease [international renal interest society (IRIS) stage IV] and one (mesenteric stage II HSA) died after 1230 days owing to a metastatic mast cell tumour. Two dogs with subcutaneous stage II HSA were still alive, 572 and 1260 days after the diagnosis. Overall, dogs receiving ADTIC had significantly longer median survival than those receiving AC (>550 days versus 142 days, respectively; P =0.011; Fig. 2). In group 1 none of the dogs was alive at 1 year after diagnosis, whereas in group 2 the 1- and 1.5-year survival rates were 66.8 and 55.8%, respectively
3.7 安全 (Safety)
对所有狗的毒性进行了评估。在第 1 组中,共评估了 77 例全血细胞计数; 中性粒细胞减少是唯一的骨髓毒性类型,发生在六只 (33.3%) 狗身上。五只狗发生 1 级中性粒细胞减少症,而一只狗患上了 3 级中性粒细胞减少症
非发热性中性粒细胞减少症。在所有狗中,中性粒细胞减少症在第一次治疗后发生,并且在没有后遗症的情况下消退。没有进一步的血液学毒性记录。在第 2 组中,共评估了 96 例 CBC;中性粒细胞减少是唯一的骨髓毒性类型,发生在 7 只 (77.7%) 狗中,发生在化疗第 1 天后 10 天。一只狗发生两次 1 级中性粒细胞减少症,五只狗发生 6 次 2 级中性粒细胞减少症,两只狗发生 4 次 3 级中性粒细胞减少症,一只狗发生 1 次 4 级非发热性中性粒细胞减少症。两只狗在每个周期后出现中性粒细胞减少症,两只狗在第三个周期后出现中性粒细胞减少症,两只狗在治疗期间只有一次中性粒细胞减少症发作。其中,1例在第二个周期后出现2级发热性中性粒细胞减少症,对症治疗后平安无事。发生中性粒细胞减少症之前给药的中位周期数为 2(范围:1-2 个周期),狗出现中性粒细胞减少症的中位周期数为 3(范围:1-4 个周期)。在所有狗中,中性粒细胞减少症在没有后遗症的情况下得到解决。
接受 ADTIC 的狗中性粒细胞减少的频率高于接受 AC 的狗(分别为 7 例中的 9 只 (77.8%) 和 6 例中的 18 例 (33.3%);P =0.046)。胃肠道毒性是两组中第二常见的不良事件,包括呕吐和食欲下降。第1组7只(38.8%)狗发生胃肠道毒性:2只狗有1级副作用(1只同时有1级中性粒细胞减少症),4只狗有2级(2只同时有1级中性粒细胞减少症),1只狗有3级毒性。在每种情况下,都记录了一次胃肠道毒性发作。在第 2 组中,三只 (33.3%) 狗出现胃肠道毒性;两只狗有 1 级(一只同时有 1 级中性粒细胞减少症),一只有 2 级毒性。胃肠道副作用的发生率在各组之间没有差异(P=1.000)。在第四个周期结束时,第 1 组中的一只狗出现脱发。没有记录到其他毒性。
All dogs were evaluated for toxicity. In group 1, a total of 77 CBCs were evaluated; neutropenia was the only type of bone marrow toxicity, occurring in six (33.3%) dogs. Grade 1 neutropenia occurred in five dogs, whereas one dog developed a grade-3
non-febrile neutropenia. In all dogs, neutropenia developed after the first treatment and resolved without sequel. No further hematological toxicities were recorded. In group 2, a total of 96 CBCs were evaluated; neutropenia was the only type of bone marrow toxicity, occurring in 7 (77.7%) dogs, 10 days after day 1 of chemotherapy. Two episodes of grade 1 neutropenia occurred in one dog, six episodes of grade 2 neutropenia occurred in five dogs, four episodes of grade 3 neutropenia occurred in two dogs and one episode of grade 4 non-febrile neutropenia occurred in one dog. Two dogs developed neutropenia after each cycle, two dogs after the third cycle and two dogs had only one episode of neutropenia during treatment. Among them, one developed a grade 2 febrile neutropenia after the second cycle, which resolved uneventfully after symptomatic treatment. The median number of cycles administered before developing neutropenia was 2 (range: 1–2 cycles), and the median number of cycles with dogs showing neutropenia was 3 (range: 1–4 cycles). In all dogs, neutropenia resolved without sequel.
The frequency of neutropenia was higher in dogs that received ADTIC than in those that received AC (7 of 9 (77.8%) versus 6 of 18 (33.3%), respectively; P =0.046). Gastrointestinal toxicity was the second most common adverse event in both groups, which consisted of vomiting and decreased appetite. Gastrointestinal toxicity occurred in seven (38.8%) dogs in group 1: two dogs had grade 1 side effects (one concurrently had grade 1 neutropenia), four dogs had grade 2 (2 concurrently had grade 1 neutropenia) and one dog had grade 3 toxicity. In every case, a single episode of gastrointestinal toxicity was recorded. In group 2, three (33.3%) dogs developed gastrointestinal toxicity; two dogs had grade 1 (one concurrently had grade 1 neutropenia) and one had grade 2 toxicity. The frequency of gastrointestinal side effects did not differ between groups (P =1.000). Alopecia occurred in one dog in group 1 at the end of the fourth cycle. No other toxicities were recorded.
4 讨论 (Discussion)
HSA 的治疗在兽医肿瘤学中仍然极具挑战性,并且由于侵袭性疾病,患有 HSA 的狗的预后很差,导致附近器官和血管的侵袭、早期转移和有限的治疗选择,从而提供持久的疾病控制。手术旨在切除所有肉眼肿瘤并防止进一步的急性出血风险,但被认为是纯粹的姑息治疗。据记载,在治疗微观疾病的过程中增加化疗可适度改善结果,据报道,中位生存时间在 6-8 个月之间,不到 10% 的狗在 12 个月时存活。
在这项研究中,我们使用多柔比星和达卡巴嗪的组合作为辅助化疗,以确定这种治疗在生物侵袭性犬 HSA 中的安全性和有效性。
这项研究表明,ADTIC 组合是可行的,并且可以在患有 HSA 的狗中每 21 天安全地给药一次,从而允许遵守预计的药物剂量和周期之间的预定间隔。所有狗都在门诊接受治疗,强调了目前描述的ADTIC方案的可行性。副作用是可逆的和可控的,中性粒细胞减少是主要毒性。一只狗在第二个周期后出现发热性 2 级中性粒细胞减少症,一只狗在第三个周期后出现无症状的 4 级中性粒细胞减少症;然而,他们都在支持性治疗下康复,随后的剂量减少被认为是没有必要的。值得注意的是,没有一只狗因中性粒细胞减少而患上败血症。
尽管在接受联合化疗的狗中预防性使用抗生素仍存在争议,但给予克拉维酸增强阿莫西林可能有效减少中性粒细胞减少发作和相关不良反应。
总体而言,胃肠道毒性(呕吐和食欲不振)的发生率较低,第1组和第2组之间没有观察到显著差异。我们假设标准止吐药物加 maropitant 可预防 3-4 级呕吐的发生。更长的maropitant给药时间(连续超过5天)可能会进一步减少呕吐的发生。
抗肿瘤药物剂量调整的具体指南在兽医肿瘤学中尚未标准化;然而,对于经历中度至重度剂量限制性毒性(即 3-4 级毒性)的患者,通常建议将后续剂量减少 20-25%,例如中性粒细胞减少或呕吐.32 过度毒性也更有可能增加治疗相关成本,有可能失去所有者的依从性,并且至少但不是最后一次,对患者的生存产生负面影响。另一方面,抗癌细胞毒性治疗可实现的最大益处需要对剂量强度的承诺;缺乏或降低剂量密度有可能对癌症治疗有害,特别是在已知具有高生长分数潜力的肿瘤疾病中.33,34 当然,最佳剂量强度需要治疗监测,以便根据患者在有效治疗期间维持高质量生活 (QoL) 的能力减少或增加剂量。在上述案例中,临床医生密切监测临床体征和详细的所有者信息,结果没有缺乏依从性。狗完全康复,血液学异常得到解决,无需住院治疗,主人也没有感觉到生活质量持续下降。因此,我们选择在下一个周期时不减少化疗剂量,从而对化疗方案的预期SDI没有影响。这种毒性在治疗期间没有复发,可归因于可能增强化疗毒性的短暂性和未确诊的合并症、个体对化疗的一定程度的耐受性、所有者对胃肠道体征的适应或这些的组合。我们的方法基于这样一种思想,即剂量减少不应仅基于毒性程度,而应根据更广泛的变异来决定,例如癌症进展的风险、临床症状和所有者的依从性。此外,在我们的研究中也没有观察到累积毒性;事实上,血液学异常是可逆的,并且在接下来的治疗周期中毒性程度(胃肠道血液学)没有增加。
这些结果与最近的出版物不同,该出版物报道了使用由DAV.15组成的联合化疗方案,在该研究中,与化疗相关的副作用是显着的,包括一些高级别的血液学和胃肠道毒性事件。此外,由于化疗相关的毒性,近 20% 的狗的方案被终止;然而,没有发生与治疗相关的死亡15。
The treatment of HSA continues to be extremely challenging in veterinary oncology, and prognosis for dogs with HSA is poor as a result of aggressive disease, leading to invasion of nearby organs and vessels, early metastasis and limited treatment options providing durable disease control. Surgery is designed to remove all macroscopic tumours and prevent further risk of acute haemorrhage, but is considered purely palliative. The addition of chemotherapy in an effort to treat microscopic disease has been documented to provide a modest improvement in outcome, with reported median survival times in the range of 6–8 months and less than 10% of dogs being alive at 12 months.1,2
In this study, we used a combination of doxorubicin and dacarbazine as adjuvant chemotherapy to determine the safety and efficacy of this treatment in biologically aggressive canine HSA.
This study showed that the ADTIC combination is feasible and can be safely administered every 21 days in dogs with HSA, thereby allowing compliance with projected drug doses and scheduled intervals between cycles. All dogs were treated on an outpatient basis, stressing the feasibility of the presently described ADTIC regimen. Side effects were reversible and manageable, with neutropenia being the primary toxicity. One dog experienced febrile grade 2 neutropenia after the second cycle and one asymptomatic grade 4 neutropenia after the third cycle; however, they both recovered with supportive care, and subsequent dose reductions were not considered necessary. Notably, none of the dogs developed sepsis due to neutropenia.
Although the prophylactic use of antibiotics in dogs receiving combination chemotherapy is still controversial, it is possible that the administration of clavulanate-potentiated amoxicillin was effective in reducing the neutropenic episodes and the related adverse effects.
Overall, the incidence of gastrointestinal toxicity (vomiting and loss of appetite) was low, and no significant differences were observed between groups 1 and 2. We assume that the standard antiemetic medication with maropitant prevented the onset of grades 3–4 emesis. It is possible that a longer maropitant administration (over 5 consecutive days) might have reduced the occurrence of vomiting further.
Specific guidelines for dose adjustments of antineoplastic agents are not standardized in veterinary oncology; however, a 20–25% reduction is commonly recommended for the subsequent dose in patients experiencing moderate–severe dose-limiting toxicity (i.e. grade 3–4 toxicities), such as neutropenia or emesis.32 Excessive toxicity is also more likely to increase treatment-associated costs, has chances of losing owners’ compliance and, least but not last, to negatively affect patients’ survival. On the other hand, the greatest benefit achievable with anticancer cytotoxic therapy requires a commitment to dose intensity; lack of or reduced dose density has the potential to be detrimental in cancer treatment, especially in neoplastic diseases known to have the potential of high growth fractions.33,34 Of course, optimal dose intensity demands therapeutic monitoring in order to either reduce or increase doses based on the patient’s capacity to maintain a high quality of life (QoL) during effective therapy. In the mentioned cases, a close monitoring of clinical signs by the clinicians and detailed owners’ information resulted in no lack of compliance. Dogs recovered completely and haematological abnormalities resolved without requiring hospitalization and with no perception of durable decline in QoL by the owner. We therefore elected not to reduce chemotherapy doses at the time of the following cycle, resulting in no effect on the intended SDIs of the chemotherapy protocol. Such toxicities did not recur during treatment, being attributable to transient and undiagnosed comorbidities that might have enhanced chemotherapy toxicities, a degree of individual tolerance to chemotherapy, adaption of the owner to gastrointestinal signs or a combination of these. Our approach was based on the thought that dose reductions should not be solely based on the degree of toxicity, but decided on a broader spectrum of variants such as risk of cancer progression, presenting clinical signs and owner compliance. Moreover, cumulative toxicity was also not observed in our study; in fact, haematological abnormalities were reversible and the degree of toxicity (either hematological of gastrointestinal) did not increase in the following treatment cycles.
These results differ from a recent publication that reported the use of combined chemotherapy protocol consisting of DAV.15 In that study, chemotherapy-related side effects were notable, including several high-grade hematologic and gastrointestinal toxic events. Moreover, almost 20% of the dogs had their protocol discontinued as a result of chemotherapy-related toxicities; however, no treatment-related deaths occurred.15
这可能有多种解释。在这项研究中,达卡巴肼的总预期剂量分为每天五次推注,而在 DAV 研究中,这是在 8 小时内作为单剂量给药;事实上,有人认为,与缓慢的静脉输注相比,每日达卡巴嗪静脉推注可能会降低胃肠道毒性,而不会产生负面影响.16,28 此外,长春新碱未在 ADTIC 狗中给药,可能降低胃肠道毒性的风险。还应该注意的是,第 2 组中的大多数狗没有临床晚期,而在 DAV 研究中,狗最有可能患有 III 期疾病;患有晚期疾病的狗很容易出现体能状态下降,可能导致对化疗毒性的易感性增加35。
在我们的研究中没有发现临床心脏毒性的证据。这一发现可归因于有关心脏功能的入组标准、研究中的狗数量有限和/或施用的多柔比星治疗数量少,未达到心脏毒性的累积剂量。
尽管这项研究的规模很小,但我们的结果表明,在转移控制和生存方面,特别是对于II期HSA,使用ADTIC治疗生物侵袭性犬HSA比AC更具优势。在第 1 组中,83% 的狗(AC 方案)因 HSA 进展而被安乐死,MST 为 142 天,而在第 2 组(ADTIC 方案)中,44.4% 的狗因肿瘤相关原因死亡,MST 为 >550 天 (P =0.011);此外,在第 2 组中,分别有 66.8% 和 55.8% 的患者实现了 1 年和一年半的生存率,而第 1 组没有患者达到一年生存率。从我们的角度来看,这些数据可能是支持ADTIC对患者生存率进一步获益的最相关数据,并且还可能表明,如果在没有宏观转移性疾病的情况下开始化疗,相当一部分患有生物侵袭性HSA的狗可能仍然有良好的结果。然而,这些数据应谨慎解释,因为它可能因第 2 组中登记的狗数量较少而存在偏差,尽管存在争议,但与其他内脏位置相比,肾脏和皮下 HSA 的侵袭性生物学行为可能较小.4,36 话虽如此,必须承认直径最长 >6 cm 的皮下 HSA 与肿瘤进展时间和生存时间较短显着相关4 在这项研究中,三个皮下 HSA 分别测量了 8、6.5 和 12 cm,支持攻击性行为和发生转移性疾病的可能性增加。此外,其中一只狗在区域淋巴结中患有转移性疾病,进一步支持了攻击性生物学行为。
关于TTM,在研究期间,接受AC治疗的狗中有66.6%发生远处转移,而接受ADTIC治疗的狗中有44%发生远处转移。与生存期一样,如果将ADTIC用作辅助一线治疗策略,则TTM显著延长(P=0.021)。这一发现可能是由于几个原因造成的。虽然环磷酰胺和达卡巴肼都是烷化剂,但由于不同的药代动力学特征、脂质溶解度、膜转运特性和能够修复DNA上烷基化位点的特异性酶促反应,它们的抗肿瘤活性差异很大.16,37环磷酰胺干扰RNA的DNA复制和转录,从而导致核酸功能的破坏.37达卡巴肼通过烷基化起作用, 16 由于优化治疗策略以最大限度地提高疗效同时限制毒性的问题具有临床意义,因此我们通过直接比较环磷酰胺和达卡巴嗪进一步研究了两种采用方案的剂量强度。与环磷酰胺相比,达卡巴嗪具有更大的个体分数剂量强度,最终导致与多柔比星联合使用时更高的 SDI。研究中包括的所有狗都接受了预定剂量,无需减少剂量;因此,接受ADTIC治疗的狗接受了更强烈的化疗,可能导致更长的TTM和生存期。
此外,除了其细胞毒性活性外,达卡巴嗪已被证明在小鼠中具有抗转移特性,其潜在机制与其增强肿瘤免疫原性的能力有关.38,39在这项研究中,用ADTIC治疗的狗具有更长的TTM,这可能反映了达卡巴嗪抑制转移扩散的能力,或者是因为研究的样本量小。
This could have multiple explanations. In this study, the total intended dose of dacarbazine was divided into five daily boluses, whereas in the DAV study this was administered as a single dose over 8-h infusion; in fact, it has been suggested that daily dacarbazine IV boluses may cause reduced gastrointestinal toxicity than slow IV infusions, without negatively affecting antitumour activity.16,28 Moreover, vincristine was not administered in ADTIC dogs, possibly reducing the risk of gastrointestinal toxicity. It should also be noted that the majority of dogs included in group 2 presented with no advanced clinical stage, whereas in the DAV study dogs were most likely to have stage III disease; dogs with advanced disease could easily have reduced performance status, potentially leading to enhanced susceptibility to chemotherapy toxicity.35
No evidence of clinical cardiotoxicity was noted in our study. This finding could be attributed to the entry criteria with regard to cardiac function, the limited number of dogs in the study and/or the low number of doxorubicin treatments administered not reaching the cumulative dose for cardiotoxicity.
Despite the small size of this study, our results document an advantage in the use of ADTIC over AC for the treatment of biologically aggressive canine HSA in terms of metastatic control and survival, particularly for stage II HSA. In group 1, 83% of dogs (AC protocol) were euthanized because of HSA progression with an MST of 142 days, whereas in group 2 (ADTIC protocol) 44.4% of dogs died because of tumour-related causes with an MST >550 days (P =0.011); moreover, in group 2, the one and one-and-a half-year survival was achieved in 66.8 and 55.8%, respectively, whereas none of the patient reached one-year survival in group 1. From our perspective, these data are probably the most relevant supporting the benefit of ADTIC on patients’ survival further, and may also suggest that a notable proportion of dogs with biologically aggressive HSA may still have a good outcome, if chemotherapy is started in the absence of macroscopic metastatic disease. However, these data should be interpreted carefully as it may be biased by the small number of dogs enrolled in group 2 and, although debated, to the potentially less aggressive biological behaviour of renal and subcutaneous HSA compared with other visceral locations.4,36 This being said, it must be acknowledged that subcutaneous HSA with the longest diameter >6 cm has significantly been associated with a shorter time to tumour progression and survival time than smaller tumours.4 In this study, the three subcutaneous HSA measured 8, 6.5 and 12 cm, respectively, supporting the aggressive behaviour and the increased likelihood of developing metastatic disease. Also, one of these dogs had metastatic disease in the regional lymph node, further supporting the aggressive biological behaviour.
Concerning TTM, 66.6% of the dogs treated with AC developed distant metastasis during the study compared with 44% of the dogs treated with ADTIC. Like survival, TTM was significantly longer (P =0.021) if ADTIC was used as adjuvant first-line treatment strategy. This finding may be due to several reasons. Although both cyclophosphamide and dacarbazine are alkylating agents, their antitumour activity differs considerably because of different pharmacokinetic features, lipid solubility, membrane transport properties and specific enzymatic reactions capable of repairing alkylation sites on DNA.16,37 Cyclophosphamide interferes with DNA replication and transcription of RNA, thereby resulting in the disruption of nucleic acid function.37 Dacarbazine acts by means of alkylation, antimetabolite activity as a purine precursor, and interaction with sulfhydryl groups in proteins.16 Because the issue of optimizing the treatment strategy to maximize efficacy while limiting toxicity has clinical implications, here we further investigated dose intensity of both adopted protocols by directly comparing cyclophosphamide and dacarbazine. Dacarbazine has greater individual fractional dose intensity when compared with cyclophosphamide, ultimately leading to a greater SDI in combination with doxorubicin. All dogs included in the study received the scheduled doses without any need for dose reduction; therefore, ADTIC-treated dogs received a more intense chemotherapy, possibly leading to longer TTM and survival.
Furthermore, besides its cytotoxic activity, dacarbazine has been demonstrated to have antimetastatic property in mice, the underlying mechanism being related to its capacity to enhance tumour immunogenicity.38,39 In this study, dogs treated with ADTIC had a longer TTM, which may either reflect the capacity of dacarbazine to inhibit metastatic spread or be because of the small sample size of the study.
这项研究的局限性包括缺乏随机化、病例数少、肿瘤部位起源不同和缺乏尸检。尽管皮下和内脏 HSA 已被描述为具有侵袭性生物学行为,1,3,4 对肠系膜 HSA 知之甚少.2 患有肠系膜 HSA 的狗被纳入本研究。此外,第 2 组中的两只狗在发生远处转移后接受了抢救方案,可能有助于提高存活率。
此外,两种治疗方案(AC 与 ADTIC)在不同的诊所提供,而不是在每个地点提供任何一种治疗,这可能导致选择偏倚。然而,对各组的分析证实,除了入组的患者数量外,这些组没有显着差异,这表明样本是同质的。
总而言之,ADTIC组合耐受性良好,可以延长具有生物侵袭性HSA的狗的TTM和生存时间,尤其是在就诊时没有转移的情况下。
Limitations of this study include lack of randomization, low number of cases, different tumour site origin and lack of necropsy. Although subcutaneous and visceral HSA have been described to have an aggressive biological behaviour,1,3,4 little is known about mesenteric HSA.2 A dog with mesenteric HSA was included in this study. In addition, two dogs included in group 2 received a rescue protocol after having developed distant metastases, possibly contributing to increased survival.
Also, the two treatment options (AC versus ADTIC) were offered at different clinics, instead of offering either treatment at each location, possibly leading to selection bias. However, the analysis of the groups confirmed that these did not have significant differences, except for the number of patients enrolled, suggesting that the samples were homogeneous.
To conclude, the combination ADTIC was well tolerated and may prolong TTM and survival time in dogs with biologically aggressive HSA, especially if not metastatic at presentation.
5 Reference
- [30] The calculation of actual or received dose intensity: a comparison of published methods. Journal of Clinical Oncology 1991
- Doxorubicin chemotherapy for presumptive cardiac hemangiosarcoma in dogs 2014 阿霉素化疗治疗狗的推定性心脏血管肉瘤
- Comparison of doxorubicin-cyclophosphamide with doxorubicin-dacarbazine for HSA 2017 多柔比星-环磷酰胺与多柔比星-达卡巴肼辅助治疗犬血管肉瘤的比较
- [b] Hill’s Neoplasia Diet, Hill’s Pet Nutrition, Topeka, KS
- Nutritional Management of Patients with Neoplasia 小动物肿瘤患者的营养管理